Ask an Oceanographer: Why is oxygen saturation greater than 100%?
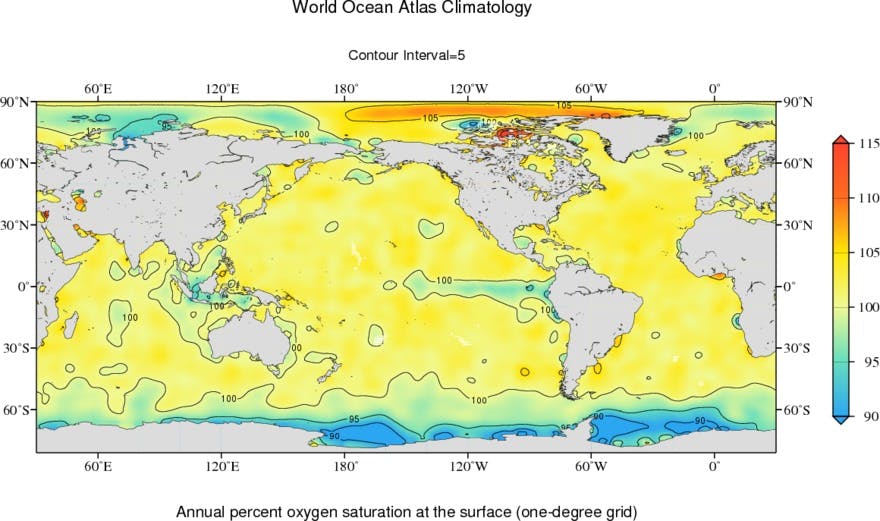
Figure from NOAA / NCEI World Ocean Atlas.
Scoot’s software developers have done a lot of work to integrate and visualize all of the data streams coming off of our aquaculture customers’ farm sites. For some, building the SeaState dashboard is their first experience working with ocean data, and the consensus is that the ocean is fascinating. This may be old news to the oceanographers on our team, but they relish the opportunity to talk shop. In this post we discuss one question that came in: “How can oxygen saturation in seawater be greater than 100%?”
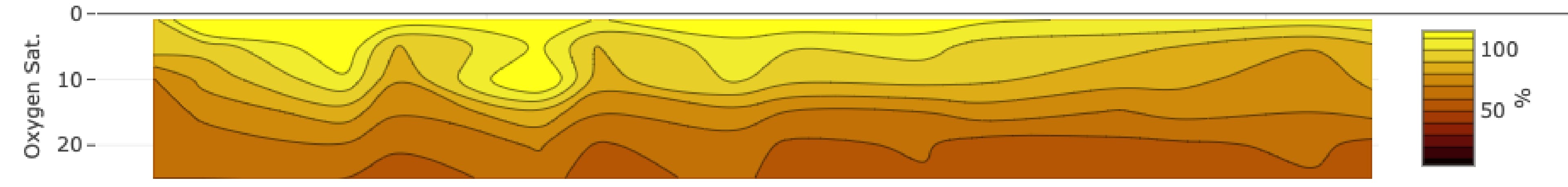
An oxygen profile from SeaState, showing oxygen saturation with depth on the y-axis and time on the x-axis. Surface oxygen saturation is above 100%.
To start, let’s consider how oxygen enters, exits, and moves around in the ocean. The sea surface is a really dynamic boundary layer where oxygen is constantly in flux between the ocean and atmosphere. If the ocean at a given location has a low oxygen concentration, oxygen moves from the atmosphere into seawater, and vice versa. Once oxygen is dissolved in seawater, it gets distributed deeper into the ocean by diffusion (very slow) and physical mixing (faster). But there’s also a biological component; organisms that photosynthesize (phytoplankton, seaweeds) add oxygen to seawater, while all of the critters that don’t photosynthesize consume oxygen. In fact, phytoplankton generate roughly 50% of the oxygen we breathe.
Oxygen saturation is a ratio of how much oxygen is dissolved in water to how much can be dissolved at a given temperature (note that the amount of oxygen that can be dissolved in seawater also depends on the salinity and pressure, but oxygen saturation is calculated based on temperature). Colder water can dissolve more oxygen than warmer water (see the figure below).
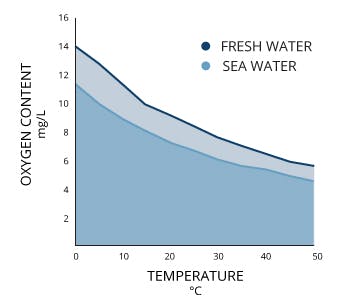
Dissolved oxygen decreases at higher temperatures (Source).
If you take a water mass that has 100% oxygen saturation and warm it up, it will have an oxygen saturation that is greater than 100% (it becomes “supersaturated”). The same thing happens if there is a phytoplankton bloom that generates more oxygen. Over time a supersaturated water mass will offgas oxygen back into the atmosphere, but this exchange doesn’t happen instantaneously.
On the flip side, we sometimes see low oxygen waters at the surface. This is typically from the wind-driven upwelling of deeper waters to the surface ocean. Deeper ocean waters have lower oxygen content because they haven’t been in contact with the atmosphere and there isn’t any photosynthesis to produce oxygen biologically. In fact, marine organisms in deep waters are constantly removing oxygen through the respiration of organic matter.
This matters a lot to aquaculturists because low oxygen levels can be lethal to fish. For salmon, oxygen saturation below about 50% is cause for concern. Unfortunately, marine pollution and climate change are causing a global “deoxygenation” of the ocean (Breitburg et al. 2018). This makes it all the more crucial for fish farmers to have access to ocean forecasts, so they can prepare for and mitigate the impact of low oxygen events.
Do you have a question for our oceanographers? Send it to info@scootscience.com and subscribe to our mailing list to get more ocean content in your inbox.